National Medicines Policy - consultation on the revised NMP
Overview
Australia’s National Medicines Policy
The Department of Health and Aged Care is inviting stakeholders to provide further feedback on a revised draft of the National Medicines Policy (NMP).
Finalisation of the NMP Review was extended until after the recent Federal Election in May 2022.
The Minister for Health and Aged Care, the Hon. Mark Butler MP has reappointed Professor Michael Kidd AM as the sole reviewer to complete the Review and provide a final report to Government later this year.
Provide your feedback on the draft National Medicines Policy
Stakeholders will have the opportunity to engage and provide feedback on the revised draft of the 2022 NMP through a range of channels. The diverse perspectives, experience and knowledge of all stakeholders is highly valued and will contribute to the report to Government and finalisation of the 2022 NMP.
The revised draft National Medicines Policy 2022 and supporting documents are available at Related Documents at the bottom of this page.
The consultation survey will be open for six weeks from 17 August 2022 until 27 September 2022 at 11:59 pm.
The online consultation survey is accompanied by targeted consultations and an open public stakeholder forum that will be scheduled during September 2022.
For enquiries about the Review please email NMP@health.gov.au.
What we’ve asked
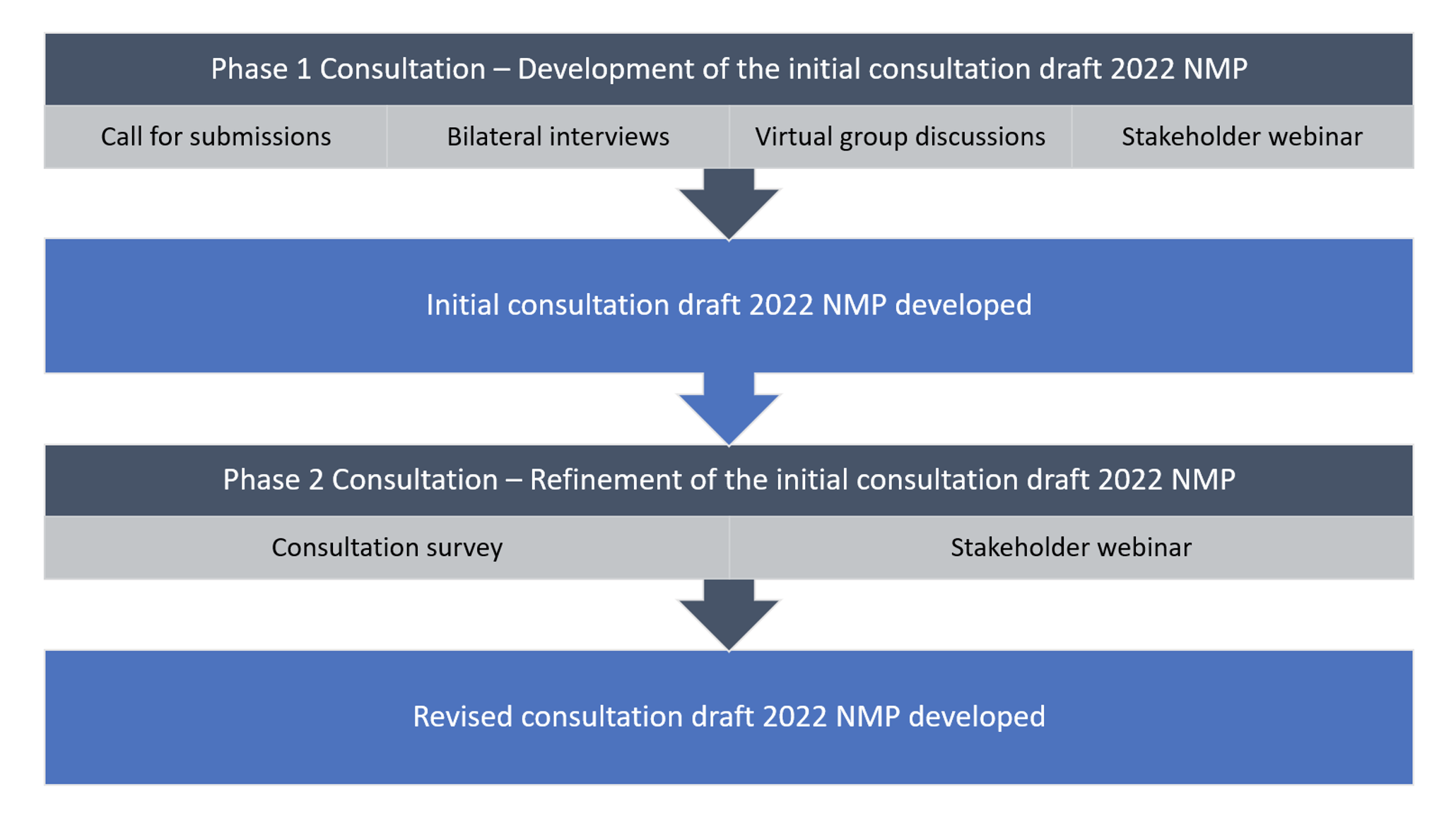
The Committee undertook extensive stakeholder engagement to ensure that a range of issues and diverse experiences informed the refresh of the NMP as a high-level policy framework. The figure below summarises the consultation phases that were completed between 30 August 2021 to 2 March 2022.
What we’ve heard
While respondents broadly agreed with most components of the initial consultation draft 2022 NMP, a summary of feedback highlighted the need to:
- Ensure consistency in the language and phrasing to strengthen readability. This included suggestions to increase the use of person-centred language, and to use clearer language to support the understanding of each of the Policy’s components. This was commonly suggested for the Policy’s aim, and the descriptions of the Policy’s principles and Central Pillars.
- Increase specificity and detail throughout the Policy. This was commonly suggested with reference to the Central Pillars, with calls for greater detail on how the Pillars would be implemented, and the roles and responsibilities of all partners.
- Provide more explicit linkage between the NMP’s components. For example, some respondents suggested that there be more explicit linkage between the Policy’s principles with the Central Pillars, governance and evaluation components (including reporting mechanisms).
What we’ve done
The Committee considered the best way to reflect stakeholder feedback and developed a revised consultation draft 2022 NMP (revised draft 2022 NMP). The Committee also developed a Summary Stakeholder Consultation Report to:
- Provide recommendations based on stakeholder feedback and their own expert experience, and
- Address feedback that was out of scope for the high-level Policy framework refresh.
This report is available (see Related Documents at the bottom of this page) and can be read in tandem with the revised consultation draft 2022 NMP.
New and updated sections
The Committee have added and updated sections of the NMP. The table below provides a snapshot of changes the Committee have made. The inclusion of this table is intended to assist in directing feedback.
|
Section |
New/Updated |
Change |
|
Vision |
New |
New addition reflecting stakeholder calls for a vision statement. |
|
Aim |
Updated |
Updated reflecting stakeholders calls for clearer aim and connections with content of Policy. |
|
Scope |
Updated |
Updated to include reference to ensure distinction between complementary medicines and Aboriginal and Torres Strait Islander traditional medicines; Includes reference to medical devices and medicines-related services. |
|
Achieving the vision and aim through partnerships |
New |
New additions which include:
|
|
Principles |
Updated |
Updated for clarity. |
|
Enablers |
Updated |
Updated for clarity. |
|
Central Pillars |
Updated |
Updated for clarity. |
|
Making the partnership work |
New |
New addition to emphasise the importance of a partnership approach to the NMP; The key responsible partner tables which were previously under each relevant Pillar can now be found in this section (pages 16-21). |
|
Governance framework |
Updated |
Updated for clarity - strengthened reference to partnership approach and clarity of purpose and is now referred to as a Governance framework. |
|
Implementation |
Updated |
Updated for clarity. |
|
Evaluation |
Updated |
Updated for clarity. |
Why we are consulting
The diverse perspectives, experience and knowledge of all stakeholders is valued and will contribute to the report to Government and finalisation of the 2022 Policy.
Consultation Information
The revised consultation draft 2022 National Medicines Policy (see Related Documents at the bottom of this page) is open for consultation via the Consultation Survey. The survey will close at 11.59 pm on Tuesday 27 September 2022. All responses must be provided through the survey.
Please contact NMP@health.gov.au for any queries related to the survey.
Targeted consultation sessions
Six targeted Webex consultation sessions are scheduled for a range of key stakeholder groups as presented below. Please be aware that due to the ongoing, high level of COVID transmission across the community, these sessions will be hosted via Webex. Please also note that Webex sessions will be recorded so that all feedback is captured and considered appropriately.
- Session A – Prescribers
- 1:30–2:30 pm Wednesday 24 August 2022
- Session B – Aboriginal and Torres Strait Islanders
- 10:00–11:00 am Thursday 25 August 2022
- Session C – Consumers
- 3:00–4:00 pm Monday 5 September 2022
- Session D – Medicines industry
- 2:30–3:30 pm Thursday 8 September 2022
- Session E – Pharmacy sector
- 3:00–4:00 pm Thursday 15 September 2022
- Session F – States and territories
- 2:30–3:30 pm Wednesday 21 September 2022
To register interest, please email NMP@health.gov.au (no later than two days prior to the session, if possible) with the subject indicating which session you wish to attend, for example “EOI Session C - Consumers”, and provide:
- your full name
- organisation (if applicable)
- nominated email address (for invitation)
You will then be sent a meeting invitation with the Webex details (including alternate dial‑in number, if required) the day prior to the session.
Stakeholders are encouraged to register for the session that most represents their area of interest, rather than registering for multiple sessions.
If you are unavailable to attend your preferred session at the allotted time, then you are encouraged to either attend another session or the broad stakeholder forum.
Open Stakeholder forum – registration details
Professor Michael Kidd AM, Deputy Chief Medical Officer and Principal Medical Advisor will facilitate an open stakeholder forum via Webex on 12 September 2022 between 3:00–5:00 pm.
To register interest, please email NMP@health.gov.au with the subject “EOI Open Forum” and provide your full name, organisation (if applicable) and nominated email address. You will then be sent a meeting invitation with the Webex details (with alternate dial‑in number, if required).
About the Survey
The survey is structured into 6 parts. Each section of the survey includes a preamble highlighting additions and amendments made by the Committee, with an example of what has changed, followed by the questions.
The survey sections and the number of questions include:
- Section 1. Privacy information
- Section 2. Introduction
- Section 3. Vision, aim, scope principles and enablers
- Section 4. Central Pillars
- Section 5. Partnerships – achieving the NMP’s vision and aim, includes
- Key responsible partners
- Governance
- Implementation
- Evaluation
- Section 6. General Comments
If you wish, you can complete the survey in stages by using the ‘save and return’ feature. At the bottom of each survey page, you can select the 'Save and come back later...' button. You will then be asked to provide an email address. A unique link will be emailed to you that will allow you to return where you left off. Email addresses entered for this purpose are not saved with your responses to the survey.
You can also choose the order in which you complete the sections of the survey. You will be returned to the contents page after you have completed the answers to each section. You will need to answer 'required' questions before you can submit your response.
How will responses be used?
The feedback gained through this process will be collated and used to support finalisation of the 2022 National Medicines Policy.
What happens next
Professor Michael Kidd AM FAHMS, Deputy Chief Medical Officer and Principal Medical Advisor will facilitate an open stakeholder forum via Webex on 20 September 2022 between 1:00 – 3:00 pm.
To register your interest in attending the open stakeholder forum, please email NMP@health.gov.au being sure to provide your full name, company (if applicable) and email address with the subject “Open Forum”.
Six targeted Webex consultation sessions will also be scheduled over coming weeks for a range of key stakeholder groups, including:
- Consumers
- Aboriginal and Torres Strait Islander representatives
- Medicines industry
- Pharmacy sector
- Prescribers, and
- States and territories.
Further information on timing and how to register interest will be published here shortly.
Audiences
- Anyone from any background
Interests
- Health technology
- Pharmaceutical benefits
- Prescription drugs
- Non-prescription medicines
- Strategic Policy

Share
Share on Twitter Share on Facebook