Draft National Clinical Quality Registry Strategy Consultation
Overview
Draft National Clinical Quality Registry Strategy
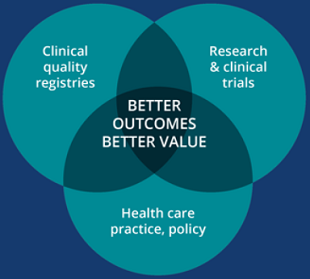
The draft National CQR Strategy aims to drive continuous improvements in the quality and value of health care to achieve better health outcomes for all Australians.
CQRs monitor the quality (appropriateness and effectiveness) of health care by routinely collecting and analysing clinical performance data. A mature CQR can provide clinicians, health service managers, patients and other stakeholders with ongoing, risk adjusted, benchmarked feedback on clinical practice and patient outcomes, to improve the standard of care.
The proposed vision of the Strategy is that:
National clinical quality registries are integrated into Australia’s health care information systems and systematically drive patient-centred improvements in the quality and value of health care and patient outcomes, across the national health care system.
The draft Strategy was developed by the Australian and state/territory governments, under the auspices of the Australian Health Ministers’ Advisory Council (AHMAC), working closely with the Australian Commission on Safety and Quality in Health Care and key stakeholders. Its development has been guided by an Expert Advisory Group. AHMAC has endorsed the draft Strategy for consultation.
Consultation process – how to have your say
On behalf of AHMAC, the Australian Government Department of Health (the Department) invites you to share your views on the draft National CQR Strategy.
The Consultation Hub includes a list of questions on the Strategy and space to provide answers. You can also upload a submission to the Consultation Hub, rather than proceed through the questions.
Important Information - Privacy and Your Personal Information
Your personal information is protected by law, including the Privacy Act 1988 and the Australian Privacy Principles (APP), and is being collected by the Department, via the Consultation Hub, for the purposes of conducting a consultation process in relation to the draft National Clinical Quality Registry Strategy.
The Department will collect your personal information at the time that you provide a submission, unless you choose to make a submission anonymously, and you are not reasonably identifiable from the information provided in your submission.
If you consent, the Department may, at its discretion, publish part or all of your submission on the Department’s website. If your submission is published, the Department may identify you as the author of the submission, if you consent to being identified.
Submissions which have been published on the Department’s website can be accessed by the general public, including people overseas.
You should not include information in your submission about another individual who is identified, or reasonably identifiable. If you need to include information about another individual in your submission, you will need to inform that individual of the contents of this notice, and obtain their consent to the Department collecting their personal information.
You can get more information about the way in which the Department will manage your personal information, including our privacy policy, at http://www.health.gov.au/internet/main/publishing.nsf/Content/privacy-policy.
The APP privacy policy contains information about:
- how you may access the personal information the Department holds about you and how you can seek correction of it; and
- how you may complain about a breach of
- the APPs; or
- a registered APP code that binds the Department; and
- how the Department will deal with such a complaint.
You can obtain a copy of the APP privacy policy by contacting the Department by telephone on (02) 6289 1555 or freecall 1800 020 103 or by using the online enquiries form at www.health.gov.au.
Consultation report
At the end of the consultation process a report will be developed, summarising input and identifying key themes raised during the consultation. The consultation report will inform the development of the Strategy and will be posted on the Department's website at
http://www.health.gov.au/internet/main/publishing.nsf/Content/Draft_National_%20CQR_Strategy
Portal closing date
The portal will be open for submissions until 20 June 2019.
Questions?
If you have questions about the process, please email CQRpolicy@health.gov.au
Why your views matter
We aim to ensure that all interested stakeholders have the opportunity to be involved in the consultation process and contribute to the development of the draft National CQR Strategy.
Upcoming webinar
As part of this process, the Department, on behalf of the Australian Health Ministers’ Advisory Council (AHMAC), would like to invite you to a webinar to discuss the draft national CQR strategy. This first webinar is scheduled to take place on Friday 14 June 2019, 2-4pm.
The first webinar panel will include representatives from the Department, the Australian Commission on Safety and Quality in Health Care (ACSQHC), and the Australian Institute of Health and Welfare (AIHW). This webinar will provide an overview of the draft National CQR Strategy and provide stakeholders with the opportunity to ask questions to better understand the aim of the Strategy, and for the Department to gather feedback.
Attendees will be able to log in and out of the webinar at any time during the two-hour period. To participate please follow this link http://livestream.education.gov.au/health/14june2019/. This will take you to the live webinar page.
What happens next
Input received during the consultation period will be summarised in a Consultation Report and will inform the development of the draft National CQR Strategy. The Report will then be posted on the Department's website at http://www.health.gov.au/internet/main/publishing.nsf/Content/Draft_National_%20CQR_Strategy.
For updates on the progress of the Strategy, please visit:
http://www.health.gov.au/internet/main/publishing.nsf/Content/Draft_National_%20CQR_Strategy.
Audiences
- Aboriginal and Torres Strait Islander People
- Academics
- Aged care service providers
- Biogicals
- Businesses
- Carers and guardians
- Commonwealth agencies
- Community groups
- Complementary medicines
- Contracted Service Providers
- Families
- General public
- Health professionals
- Health workforce
- Local governments
- Medical Devices & IVDs
- Men
- Non-government organisations
- Other
- Over-the-counter medicines
- Parents
- Prescription medicines
- Seniors
- State government agencies
- Women
- Young people
Interests
- Aboriginal and Torres Strait Islander health
- Children's health
- Chronic disease
- Dementia
- e-Health
- Health insurance
- Health technology
- Hospitals
- Legislation
- Medicare
- Mental health
- Organ and tissue donation
- Pharmaceutical benefits
- Policy Development
- Prescription drugs
- Regulatory policy
- Rural health services
- Strategic Policy
- Women's health


Share
Share on Twitter Share on Facebook